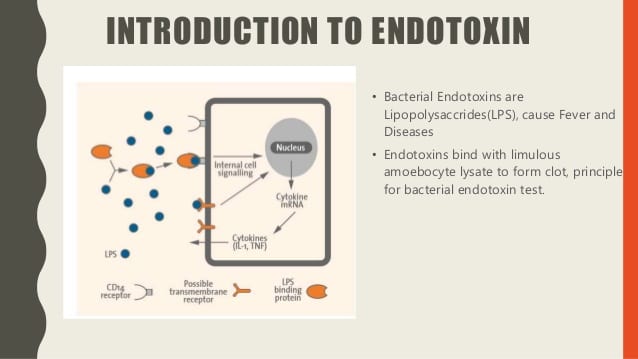
The bacterial endotoxin test is also known as the LAL test, and the bacterial endotoxin test is considered the most sensitive, as well as a specific method available for the detection and measurement of bacterial endorphins (fever). It works by generating pyrogen, a by-product of gram-negative bacteria. This bacterium causes the blood to clot in Limulus Polyphemus. The concept of coagulation is attributed to the enzymatic reaction to endotoxin by elements present in the intercellular fluid of circulating cells; amoebocytes The LAL test is approved by the Food and Drug Administration for the detection of endotoxins in medicines.
Applications of protein lysates
The phenomenon is used when estimating the level of concentration of bacterial endotoxins that may be present in or on a sample. The reaction rate usually depends on the endotoxin concentration, temperature, and pH. For the reaction to occur, particular bivalent cations must be present (protein capable of coagulation and a procoagulant enzyme system). Here’s a useful reference https://www.biochain.com/products/protein/total-protein-lysate/
Test preparation
The test should be done in a way that facilitates the prevention of microbial contamination. Contamination can cause side effects such as nausea/vomiting, hypotension, headache, chills, fever, spontaneous abortion, acute lung injury and, in the worst case, death. If necessary, the equipment should be treated to eliminate the endotoxins. The principle of the bacterial endotoxin test (BET) requires that the following details be verified before performing the analysis:
1. The equivalent used does not adsorb endotoxins
2. Check the sensitivity of the lysate and
3. Make sure that the interference factors are absent
Checking the sensitivity of the protein lysate
Prepare at least four sets of replicates containing at least three dilutions of a standard preparation. The objective is to guarantee that at least each series gives a negative result in the final dilution. Evaluate the dilution and the negative control solution, including BET water. Then the logarithmic average of the lowest endotoxin concentrations in each dilution series is calculated with a positive result.
The calculation of the antilogarithm of the averages produces the relative sensitivity of the lysate. If the sensitivity to the lysate obtained does not vary by more than a factor of 2, based on the indicated sensitivity, it is confirmed. The sensitivity of the determined lysate is then used in all tests performed using this particular protein lysate.
Lysate analysis
The sample chosen for the analysis is added to the protein lysate obtained from the hemolymph cells of Limulus Polyphemus, also known as the horseshoe crab. The crab is not killed. The principle of the endotoxin test is based on the physiological effect. Crab blood contains a type of blood cell called an arborio. Body Cells play a vital role in exposing defense mechanisms against pathogens.
Crab blood cells have granules that contain a clotting property called coagulogen. The coagulation element is released outside the body cell when it encounters bacterial endotoxin. It is believed that the resulting coagulation includes bacterial infections in the semi-closed system of the animal. Before the development of the bacterial endotoxin test, rabbits were used to analyze the pyrogen. The sample to be examined will be injected into a rabbit, after which the increase in temperature will be controlled.
The freezing and clotting method is the most sensitive and accurate procedure, with fewer false positives and negatives. In spite of its precision, it is not automatic, it consumes a lot of time and is subject to chemical and physical inhibitors, protein denaturation and pH alteration. Quantitative methods can be automated, and results can be easily calculated.
Conclusion
Although they are easy to use, these methods can be modified by analyzing blood, plasma, albumin, serum and similar substances. Both ways are sensitive to excessive turbidity. The turbidimetric method is associated with many false positives, while many compounds interact with the chromogenic way, which makes it ineffective in most cases.
type in your search and press enter
Ways on how Protein Lysate is used in Bacterial Endotoxin Test